
From the 2023 4th Quarter Pressure

Prohibited Item Risk Assessment
Introduction
What is the rationale behind having a prohibited items assessment/checklist for each hyperbaric unit? The hyperbaric safety program forms the cornerstone of every hyperbaric medicine service, offering essential guidance to identify and mitigate the potential risks and hazards associated with hyperbaric oxygen therapy. In certain circumstances, the risk cannot be eliminated (e.g., removing a wound care product), and mitigating measures must be taken to reduce the risk potential. National Fire Protection Association (NFPA) Healthcare Facilities Code 99, Appendix A for Chapter 14 Hyperbaric Facilities (NFPA 99-14) provides a flow chart that should be followed to determine the risk of patient care items that may be introduced into the hyperbaric environment. (1) The Hyperbaric Safety Program, as a standard process, should require that every patient care item introduced into the hyperbaric environment have a risk assessment completed prior to use in the chamber.
The NFPA Risk Assessment Flow Chart
The NFPA 99-14 risk assessment flow chart is a detailed process for effectively making decisions on the safety of patient care product(s) in a hyperbaric environment. (1) Products may include wound care dressings, textiles, other related products, and patient care devices.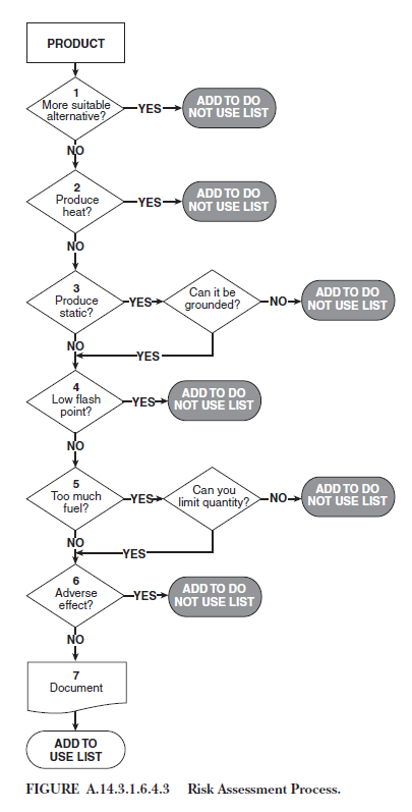 _______________________________________________________
_______________________________________________________
The risk assessment flow chart provides a methodical decision-making process that considers safety concerns such as spontaneous heat production, static electricity, flammable vapor, ignition temperature, and the total fuel load. Unfortunately, the aforementioned risk assessment tool does not provide a means for documentation of the risk assessment, nor does it attempt to quantify the significance of the risk. A.14.3.1.6.4.3 of the NFPA 99 2021 advises: "The hyperbaric facility should maintain a “use list” and a “do not use list” of items that have been evaluated for hyperbaric use.” In addition to this list, it is important to keep documentation on file explaining the risk assessment for each item. Hence, the facility-specific foundation requires an “exceptions log.”
Each area within the assessment tool should be evaluated independently. The risk assessment evaluation begins by obtaining the Safety Data Sheet (SDS) for the product or potentially prohibited item and answering several basic questions. Sometimes there is no specific SDS for wound care/dressing items. However, one can find SDS information of the contents found within the dressing or product (e.g., Xeroform is not found, but petroleum jelly is.)
Specifically, what is a potentially prohibited item? Can the item or product be removed before commencing treatment? If removal is not an option, are there viable alternatives, and what measures must be taken to minimize the associated risks? For instance, does the item generate heat, and what steps can be taken to mitigate this risk if it does?
Evaluating risks in our current practice poses difficulties because the necessary information for conducting a thorough assessment might not be readily accessible. Guidance to the following questions can be unclear:
- Understanding the purpose of the NFPA 99-14 Appendix A published flow chart
- Finding the answers to the questions that are posted in the flow chart (e.g., often answers are assumptions)
- How to proceed when information is not readily available
- Wound care dressing and product safety data sheets (SDS) routinely do not report all the information needed.
- Completing the current process does not provide the necessary detail to identify the level of risk, the exposure potential, and the consequences if the item is allowed into the chamber.
- Completion of the current process does not guarantee adequate documentation. Further effort must be invested into documenting the decision process.
In this document, we propose a scoring system, specific decision tree, and standardized evaluation process to identify and mitigate potential safety go/no go risks in the hyperbaric chamber environment.
Burman’s Risk Scoring System
Quantifying risk magnitude through a practical scoring approach.
When considering a material, dressing, disinfectant, piece of equipment, or other product for use in a hyperbaric chamber, a consistent process should be followed to ensure that any identified real risks can be effectively mitigated.
Generally, very few products are manufactured specifically for hyperbaric use or evaluated and declared effective and/or safe by manufacturers. However, in equal measure, many typical healthcare products do not present any significant risk of endangering patients, staff, or facilities.
Medical products require FDA approval prior to marketing and sale, but even with this approval, this does not apply in many cases to cover a hyperbaric medicine application. In order to meet the FDA requirements for off-label use, the medical director is required to decide both in the best interests of the patient and with due regard to safety. (2)
The exposure of patients, staff, and equipment to the hyperbaric oxygen environment will always present some risk, which should be evaluated and mitigated as much as is practically and realistically achievable.
A risk assessment process based on identified hyperbaric facility hazards should allow each item to be properly evaluated and an appropriate risk level determined, enabling the risk management team and the medical director to make an appropriate decision.
Types of Risks
Three risk categories need to be considered: fire, mechanical and physiological. Items under consideration should be evaluated to determine whether any or all of these categories apply and, if so, what the magnitude of the risk would be.
Definitions
Risk: a risk can be defined as the probability of an exposure to a hazard leading to negative consequences. This, in turn, allows each of the above three identified areas of concern to be scored, using a Likert Scale, for probability, frequency of exposure, and severity of potential consequences.
- Fire hazards: fire has been proven to be the greatest of the risks encountered in hyperbaric chambers through the review of more than 95 years of experience in this field. (3) Items introduced into the hyperbaric environment should be considered in terms of a potential source of fuel, an ignition source, and exposure to oxygen to promote and accelerate combustion.
- Mechanical hazards include anything that leads to(4):
- Undesired release of compressed gas from the chamber: even small volumes of compressed gas represent a large amount of potential energy. Should this energy be released suddenly, the effects on adjacent structures and personnel can be devastating. The release could result from failure of the vessel or the associated piping or if the chamber is modified in a manner contrary to the original code or standard of design and construction.
- Impaired access: any restriction on escape or impedance to rescue and firefighting efforts posed by the chamber creates a hazard in case of an emergency.
- Reduced visibility through the chamber: reduced or restricted vision of chamber operators reduces their effectiveness as safety monitors (e.g., an external fixator that scratches the chamber acrylic window).
- Collapse or rupture during changes in pressures: for instance, sealed or semi-sealed containers that are not adequately vented left inside the chamber will either collapse under pressure (possibly resulting in adiabatic heating of the contents and thus imposing a fire or explosion risk); or explode on resurfacing if the gas trapped within the container cannot escape during the depressurization.
- Malfunction, disruption, or inoperativeness of many standard items when placed in service under hyperbaric conditions, including the implosion of lamps and vacuum tubes (e.g., cathode ray tubes in medical monitors), overloading of fans due to a higher gas density, inaccurate operation of flowmeters, pressure gauges, and regulators.
- Physiological hazards (4): Physiological hazards for the purpose of the Risk Assessment Tool include only those arising as a result of mechanical, electrical, or other safety malfunctions. For instance:
- General hazards: these include electric shock or overheated materials leading to acute burns, CO poisoning, intoxication by products generated during the combustion of materials (e.g., cyanide and chlorine).
- CO2 intoxication: should the ventilation or air exchange system malfunction or be inadequate, CO2 levels could rise to toxic levels (due to the increased atmospheric pressure).
- Rapid depressurization of the chamber may lead to barotrauma, discomfort, and/or hypoxia. This hazard may result when overpressurization occurs, and the relief valve is activated.
- Uncomfortably high noise for the patient may occur during compression and subsequent ventilation.
- Probability of an accident: the likelihood that an incident or accident, such as combustion due to fire/explosion, mechanical failure of the chamber, or undesired physiological event, will happen as a result of exposure to the hazard
- Frequency of exposure: how often will the facility, occupant, or staff member be exposed to the hazard if no mitigation actions are taken.
- Severity of potential consequences: severity of the outcome, should an accident occur
Risk Score = probability x exposure x consequence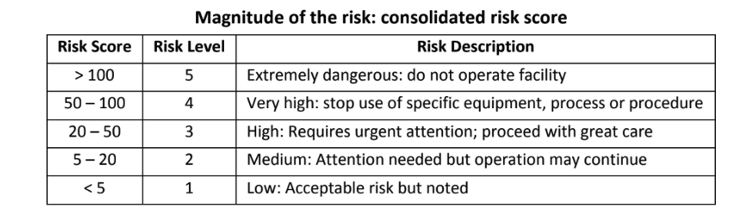
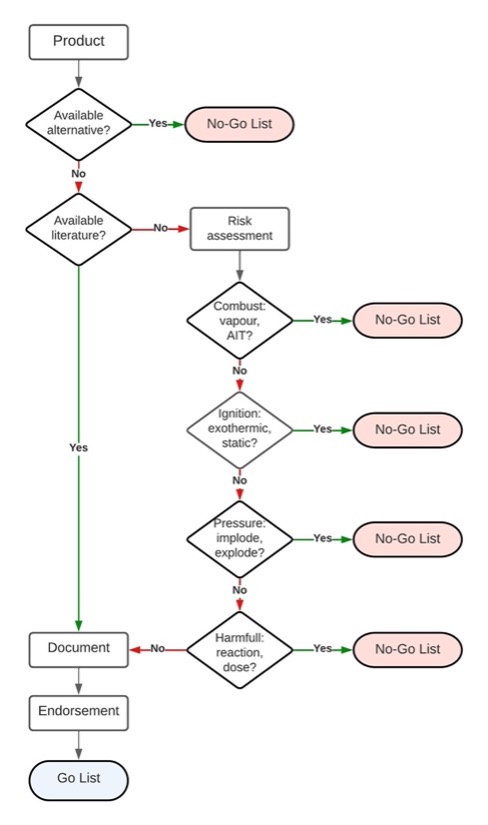
The Go No-Go Risk Assessment Tool with Burman’s Scoring System
NFPA recommends that hyperbaric facilities utilize the NFPA risk assessment flowchart to assess dressings and items that have not been evaluated or deemed safe for use in the hyperbaric chamber. However, several challenges (as described above) prevent widespread use of the NFPA risk assessment flow chart. Most importantly, completion of the published flow chart does not provide the necessary detail to identify the level of risk, the exposure potential, and the consequences if the item is allowed into the chamber.
The Go No-Go Risk Assessment Tool addresses this gap by combining the NFPA risk assessment flow chart and Burman’s Risk Scoring System. Combining the two tools with an easy-to-use interface will provide the hyperbaric facility with a robust go/no-go documentation and mitigation tool.
Example:
Wound-care medication product, applied by a physician that cannot realistically be removed prior to hyperbaric oxygen treatment.
The potential hazards are fire and a potential reaction to the product through exposure to an elevated partial pressure of oxygen. The initial risk assessment could be considered as follows:
1. Fire:
- Identifying risks:
- Fuel – The product Safety Data Sheet (SDS) should be consulted to determine whether any volatile vapors can be released at expected chamber temperatures and elevated partial pressure of oxygen (this information is not always easy to obtain – the manufacturer may need to be consulted).
- Ignition source – in the event of any potentially flammable materials, the required heat to initiate a fire is relatively low.
- Assigning scores:
- Probability of a fire: 3 (combustion possible if any flammable vapors can be released)
- Exposure: 4 (less than twice a day)
- Consequence: 5 (potentially catastrophic)
- Quantifying the risk level
- Risk level 3 x 4 x 5 = 60
- A score of 60 represents high risk - risk mitigation is needed prior to use. Ensuring no flammable vapors can be released, grounding the patient, and ensuring prohibited items are excluded from the chamber would reduce the probability of fire to a 1 (new risk score 1 x 4 x 5 = 20). In addition, a damp towel could be placed over the dressing area to further protect against any possible ignition.
2. Physiological:
- Identifying risks:
- Amended uptake of medication/dressing product.
- Quantifying the risk level:
- No possibility for any human reaction would yield a risk score of 1 x 4 x 0 = 0
- Potential reaction of medication with oxygen (not as yet been reported): 3 x 4 x 3 = 36
- A score of 36 represents a medium risk – treatment is not advised if this specific product is used.
Impression
Risk assessments and mitigation steps taken should be undertaken in writing and presented to the medical director for endorsement.
Reference:
- Technical Committee on Hyperbaric and Hypobaric Facilities (HEA-HYP). 14. Hyperbaric Facilities. In: (NFPA) National Fire Protection Association, editor. NFPA 99: Health Care Facilities Code Handbook. 2021st ed. Quincy, MA: NFPA; 2021.
- FDA. “Off-Label” and Investigational Use Of Marketed Drugs, Biologics, and Medical Devices | FDA [Internet]. 1998 [cited 2021 Feb 21]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/label-and-investigational-use-marketed-drugs-biologics-and-medical-devices
- Sheffield PJ, Desautels DA. Hyperbaric and hypobaric chamber fires: a 73-year analysis. Undersea Hyperb Med. 1997 Sep;24(3):153–64.
- Burman F. Risk Assessment Guide for Installation and Operation of Clinical Hyperbaric Facilities. 6th ed. San Antonio, Texas USA: International ATMO, Inc.; 2019.
- Burman F, Mize J, (2023). "Prohibited Item Risk Assessment ". In Worth E, Song E, (Eds.) , WoundReference. Available from: https://woundreference.com/app/topic?id=prohibited-item-assessment. Retrieved on 9/28/23.




















